Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanno, Takashi; Takeuchi, Masayuki; Otsuka, Satoshi; Kaito, Takeji
Journal of Nuclear Materials, 494, p.219 - 226, 2017/10
Times Cited Count:17 Percentile:85.35(Materials Science, Multidisciplinary)Oxide dispersion strengthened (ODS) steel cladding tubes have been developed for fast reactors. 9 chromium ODS and 11Cr-ODS tempered martensitic steels are prioritized for the candidate material in research being carried out at JAEA. In this work, fundamental immersion tests and electro-chemical tests of 9 to 12Cr-ODS steels were systematically conducted in various nitric acid solutions at 95 C. The corrosion rate exponentially decreased with effective solute chromium concentration (Cr
C. The corrosion rate exponentially decreased with effective solute chromium concentration (Cr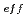 ) and nitric acid concentration. Addition of oxidizing ions also suppressed the corrosion rate. According to polarization curves and surface observations in this work, the combination of low Cr
) and nitric acid concentration. Addition of oxidizing ions also suppressed the corrosion rate. According to polarization curves and surface observations in this work, the combination of low Cr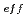 and dilute nitric acid could not prevent the active dissolution at the beginning of immersion, and the corrosion rate was high. In comparison, higher Cr
and dilute nitric acid could not prevent the active dissolution at the beginning of immersion, and the corrosion rate was high. In comparison, higher Cr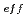 , concentrated nitric acid and addition of oxidizing ions helped to prevent the active dissolution, and suppressed the corrosion rate.
, concentrated nitric acid and addition of oxidizing ions helped to prevent the active dissolution, and suppressed the corrosion rate.
 to obtain the high U solution
to obtain the high U solution; *; Sakurai, Koji*; ; Nomura, Kazunori; *
JNC TN8400 2000-032, 98 Pages, 2000/12
Concerning the preparation of high U solution for the crystallization process and the application of UO powder dissolution to that, the effects of final U concentration, dissolution temperature, nitric acid concentration and powder size on the dissolution of UO
powder dissolution to that, the effects of final U concentration, dissolution temperature, nitric acid concentration and powder size on the dissolution of UO powder in the nitric acid where the final U concentration was
powder in the nitric acid where the final U concentration was  800g/L were investigated. The experimental results showed that the solubility of UO
800g/L were investigated. The experimental results showed that the solubility of UO decreased with the increase of final UO
decreased with the increase of final UO concentration and powder size, and with the decrease of dissolution temperature and nitric acid concentration. It was also confirmed that in the condition where the final U concentration was sufficiently lower than the solubility of U, UO
concentration and powder size, and with the decrease of dissolution temperature and nitric acid concentration. It was also confirmed that in the condition where the final U concentration was sufficiently lower than the solubility of U, UO dissolution behavior in the high U solution could be estimated with the equation based on the fragmentation model which we had already reported. Based on these experimental results, the dissolution behavior of irradiated MOX fuel in high U solution was estimated and the possibility of supplying high U solution to the crystallization process was discussed. In the preparation of high U solution for the crystallization process, it was estimated that the present dissolution process (dissolution for fuel pieces of about 3cm long) needed a lot of time to obtain a high dissolution yield, but it was shorted drastically by the pulverization of fuel pieces. The burst of off-gas at the early in the dissolution of fuel powder seems to be avoidable with setting the appropriate dissolution condition, and it is important to optimize the dissolution condition with considering the capacity of off-gas treatment process.
dissolution behavior in the high U solution could be estimated with the equation based on the fragmentation model which we had already reported. Based on these experimental results, the dissolution behavior of irradiated MOX fuel in high U solution was estimated and the possibility of supplying high U solution to the crystallization process was discussed. In the preparation of high U solution for the crystallization process, it was estimated that the present dissolution process (dissolution for fuel pieces of about 3cm long) needed a lot of time to obtain a high dissolution yield, but it was shorted drastically by the pulverization of fuel pieces. The burst of off-gas at the early in the dissolution of fuel powder seems to be avoidable with setting the appropriate dissolution condition, and it is important to optimize the dissolution condition with considering the capacity of off-gas treatment process.
; Koyama, Tomozo; Funasaka, Hideyuki
JNC TN8400 2000-014, 78 Pages, 2000/03
We investigated the factors which affected the dissolution of U and Pu to the nitric acid solution with the fragmentation model, which was based on the results of dissolution experiments for the irradiated fast reactor fuels in the Chemical Processing Facility(CPF). The equation that gave the fuel dissolution rate was estimated with the condition of fabrication (Pu ratio (Pu/(U+Pu))), irradiation (burn-up) and dissolution (nitric acid concentration, solution temperature and U+Pu concentration) by evaluating these effects quantitatively. We also investigated the effects of fuel volume ratio to the solution in the dissolver, burn-up and flouring ratio of the fuel on the f-value (the parameter which shows the diffusion and osmosis of nitric acid to the fuel) in the fragmentation model. It was confirmed that the fuel dissolution rate calculated with this equation had better agreement with the results of dissolution experiments for the irradiated fast reactor fuels in the CPF than that estimated with the surface area model. In addition, the efficiency of this equation was recognized for the dissolution of unirradiated U pellet and high Pu enriched MOX fuel. It was shown that the dissolution rate of the fuel slowed down at the condition of the high U-Pu concentration dissolution by the calculation of the dissolution behavior with this equation. The dissolution of the fuel can be improved by increasing the nitric acid concentration and temperature, but from the viewpoint of lowering the corrosion of the dissolver materials, it is desirable that the f-value is increased by optimizing the condition of shearing and stirring for the improvement of dissolution.
*; *; *; *
JNC TJ8400 2000-061, 92 Pages, 2000/03
Crystallization procedure is considered to have an advantage in recovering rather pure uranium from contaminated uranium solution and to be applicable for a new reprocessing process. It was confirmed until last year that the reprocessing process with crystallization procedure has a sufficient advantage. But the data for Pu crystallization is very rare. although it is necessary for design of the process with crystallization procedure. In this study, a beaker scale plutonium test was performed in AEA Technology Harwell Laboratory to confirm a behavior of Pu (IV) nitrate under crystallization condition. The results were examined by Mitsubishi Materials Corporation. Test item was a measurement of temperature in case of Pu (IV) nitrate crystallization or freezing of the solution in the following six parameters. (Pu(g/L):200, 100, 50, HNO (m):6, Pu valence:4). (Pu(g/L):200, 100, 50, HNO
(m):6, Pu valence:4). (Pu(g/L):200, 100, 50, HNO (m):4, Pu valence:4). Test results were as follows. (1)Pu(IV) nitrate crystallization was not observed even in the case 200g Pu/L and HNO
(m):4, Pu valence:4). Test results were as follows. (1)Pu(IV) nitrate crystallization was not observed even in the case 200g Pu/L and HNO 6M and 4M which were considered to the best condition but crystal of H
6M and 4M which were considered to the best condition but crystal of H O and HNO
O and HNO
 3H
3H O were observed. (2)Similar results were obtained for the other parameter with lower Pu concentration. (3)We can estimate that Pu(IV) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (4)The solubility data of Pu(NO
O were observed. (2)Similar results were obtained for the other parameter with lower Pu concentration. (3)We can estimate that Pu(IV) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (4)The solubility data of Pu(NO )
) - HNO
- HNO -H
-H O system was obtained.
O system was obtained.
; Miyata, Teijiro
Nihon Genshiryoku Gakkai-Shi, 36(8), p.744 - 751, 1994/00
Times Cited Count:3 Percentile:35.79(Nuclear Science & Technology)no abstracts in English
Nagaishi, Ryuji
no journal, ,
no abstracts in English